Synonymy of Pseudochazara misjai and Pseudochazara tisiphone dibra: taxonomic reassessment of an Albanian butterfly population.
Is the glass half full or half empty? An inconvenient truth.
Published online: 25.v.2025.
DOI: 10.5281/zenodo.15492634
.
.
3/ Climatic differences between the disjunct populations in Korçë and Dibër Counties
To compare the macro-climate data with high-quality, historical records, Meteoblue and NOAA's Climate Data Online are excellent resources. Meteoblue is particularly user-friendly for accessing long-term trends while NOAA provides raw historical data for professional use, including climate studies. Meteoblue offers data at a 1x1 km coordinate resolution, whereas NOAA's Climate Data Online (CDO) provides climate data at various spatial resolutions, though it generally does not offer 1x1 km resolution for all datasets.
Therefore, Meteoblue was selected to compare two habitats with healthy Pseudochazara populations at altitudes as closely comparable as possible. The habitat near Bulqizë (41.44N 20.20E) is at 1061 m asl, while in Korçë County, the habitat near Boboshtiçë (40.54N 20.77E) is at 1134 m asl.
The elevation difference of 73 meters can be adjusted for using the lapse rate, which describes how temperature decreases with altitude: (73 m/ 1000) × 6.5°C = 0.4745°C. To compare Tmax at 1134 m to 1061 m, add 0.47°C to the 1134 m value.
While both Tmax and Tmin are affected by altitude, Tmin is generally more sensitive to changes in altitude due to the cooling of the air overnight.
Bulqizë has a continental climate, characterised by cold winters with snowfall and warm summers. Boboshtiçë has a Mediterranean-continental climate, with somewhat milder winters compared to Bulqizë, but still cold enough to see some snow. Summers are warmer in Boboshtiçë. Precipitation in Boboshtiçë is generally lower than in Bulqizë and summers are generally drier in Boboshtiçë compared to Bulqizë although occasional thunderstorms can still occur.
In Table 1, a summary of climate data between the two localities is presented, while the full data including graphics (Supplementary material S1) are accessible through AWPL online.
The definitions of the climate variables can be consulted on the Meteoblue website.
Table 1. Summary of climate data between the habitats in Bulqizë and Boboshtiçë.
| 21,08 |
21,25 |
-0,17 |
21,72 |
-0,64 |
| -2,58 |
-1,17 |
-1,42 |
-0,69 |
-1,89 |
| 8,48 |
7,88 |
0,59 |
|
|
| |
|
|
|
|
|
| 4,63 |
6,09 |
-1,46 |
|
|
| 22,58 |
21,24 |
1,33 |
|
|
| 3,23 |
3,11 |
0,12 |
|
|
| |
|
|
|
|
|
| 126,42 |
60,58 |
65,83 |
|
|
| 15,58 |
12,57 |
3,01 |
|
|
| Avg. monthly snow days (d) |
3,48 |
3,23 |
0,26 |
|
|
| 5,13 |
5,42 |
-0,28 |
|
|
| 3,17 |
3,25 |
-0,08 |
|
|
| 2,93 |
2,20 |
0,73 |
|
|
| 2,61 |
1,22 |
1,39 |
|
|
| 1,60 |
0,47 |
1,13 |
|
|
| 0,12 |
0,01 |
0,11 |
|
|
| |
|
|
|
|
|
| 2,00 |
2,58 |
-0,58 |
|
|
| 8,08 |
9,83 |
-1,75 |
|
|
| 4,83 |
5,75 |
-0,92 |
|
|
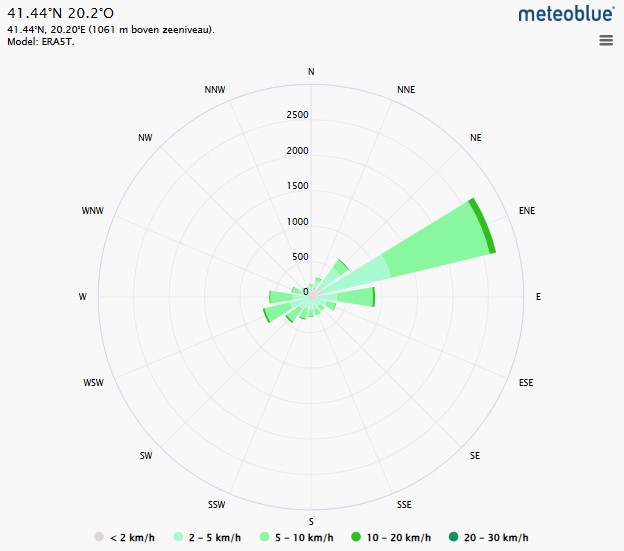 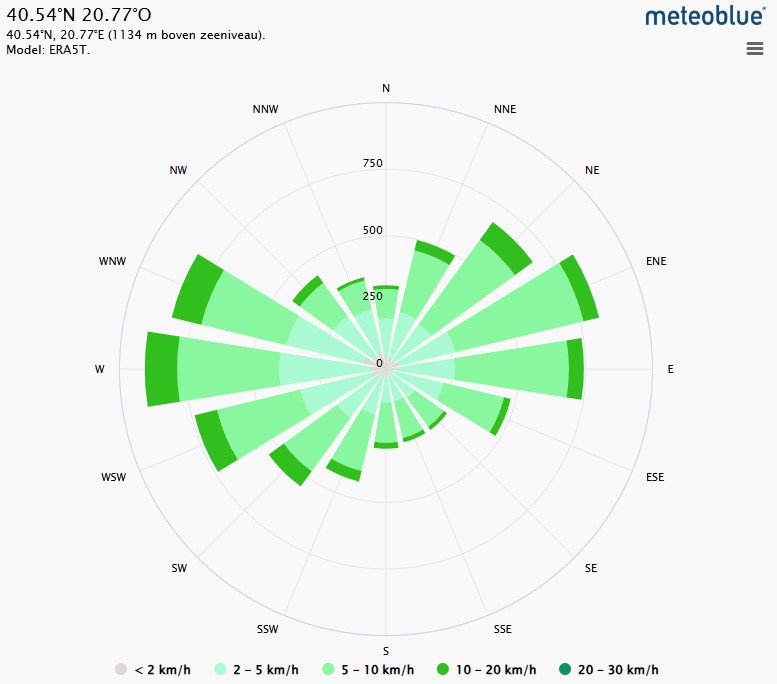 Fig. 1. The wind speed analysis table is complemented by two wind rose diagrams from Meteoblue, illustrating wind patterns: Bulqizë (left) and Boboshtiçë (right). Fig. 1. The wind speed analysis table is complemented by two wind rose diagrams from Meteoblue, illustrating wind patterns: Bulqizë (left) and Boboshtiçë (right).
5/Available data regarding androconial scales
The androconial scales of the Dibër population are illustrated in Cuvelier, Parmentier et al. (2018) and in Parmentier & Qirinxhi (2025).
In the 2018 publication, androconial scales from a single specimen, removed from two areas on the forewing, were photographed without the use of cover glass, using a calibrated 5 megapixel Dino-Lite AD- 7013MZT. These images, presented in Fig. 12c-d of Cuvelier, Parmentier et al. (2018), closely resemble the studied androconial scales of P. tisiphone specimens from southeastern Albania, as illustrated in Cuvelier & Mølgaard (2015). Comparing the androconial scales of this Dibër specimen with the figures of P. tisiphone androconial scales in Wakeham-Dawson (1997), reproduced hereunder in Fig. 2, with the kind permission of A. Wakeham-Dawson, and Fig. 1 in Wakeham-Dawson & Kudrna (2006) show a similar shape matching best with group 4 androconial scales as described in Gross (1978) and Wakeham-Dawson (1997).
7/ Current extent and quality of the available genetic data
Until now, studies on Pseudochazara species have primarily relied on COI barcoding, which, while valuable, does not encompass the full mitochondrial genome. , Verovnik & Wiemers (2016), , Dapporto et al., Appendix S1 (2022), Parmentier & Qirinxhi (2025) together with the phylogenetic tree presented in .
Furthermore, data from single nuclear genes, as well as larger studies utilizing high-throughput sequencing or full genome sequencing, are lacking.
The DNA barcode sequencing results of three specimens (RVcoll14U545, RVcoll14U546, and RVcoll14U547) are included in both Cuvelier & Marafi (2025) and Parmentier & Qirinxhi (2025). In Parmentier & Qirinxhi (2025) new COI sequences, deposited in GenBank, with accession numbers are mentioned.
A phylogenetic tree of Pseudochazara species based solely on complete COI sequences (658 bp), together with the haplotype network for the Pseudochazara group to which P. tisiphone belongs, is presented in Supplementary material S4 and Supplementary material S5, and further explored in the Discussion chapter.
8/ Addressing potential confusions and inaccuracies in the article deposited in the Figshare repository on March 2, 2025
The publication by Parmentier & Qirinxhi (2025) contains editorial oversights, inconsistencies, and inaccuracies that may lead to misinterpretations of the facts.
These issues are addressed and, where possible, clarified in the discussion section of the present publication.
9/ P timeline since Gross's 1973 description of Pseudochazara sintenisi cingovskii, including subsequent taxonomic changes and synonymies
Gross (1973) is the source of the original taxonomic description of Pseudochazara sintenisi cingovskii.
Koutsaftikis (1974) reported Pseudochazara sintenisi Stgr. from Greece for the first time. The figure of a female specimen in this publication clearly depicts an individual that was later associated with tisiphone.
Brown (1976) established P. cingovskii Gross as a new status, with the holotype described from Mt. Smolikas.
Gross (1978) discussed P. cingovskii from Yugoslav and Greek Macedonia. He did not assign the Greek population to any subspecies. The publication includes an illustration of the androconial cell of P. cingovskii from Prilep (present-day North Macedonia).
Brown [1981] described P. cingovskii tisiphone as a new subspecies.
De Prins & van der Poorten (1981) described a new species of Pseudochazara from the Drama district (northeastern Greece): Pseudochazara orestes De Prins & van der Poorten, 1981.
Hesselbarth, van Oorschot & Wagener (1995) reclassified P. cingovskii tisiphone as Pseudochazara mniszechii tisiphone stat. nov., based on a single population near Bursa (Turkey) and from Greece.
Wakeham-Dawson (1997) reclassified P. cingovskiii tisiphone as Pseudochazara mniszechii tisiphone stat. nov., independent of Hesselbarth et al. (1995).
This view is also supported by Lukhtanov (2007) in Nymphalidae: Satyrinae (Global Butterfly Names Project).
This opinion, that P. mniszechii tisiphone is the valid taxon, is also supported in in their study on partial mtCOI-sequences of Balkanic species of Pseudochazara, where COI barcoding was first used to confirm the classification.
Verovnik & Wiemers (2016), based on a deep genetic split between P. mniszechii and P. mniszechii tisiphone, elevated the latter to species level.
Cuvelier, Parmentier, Paparisto & Couckuyt (2018) reported, for the first time, a new disjunct population of P. tisiphone in Dibër County.
Dincă V., Dapporto L., Somervuo P., Vodă R., Cuvelier S., Gascoigne-Pees M., Huemer P., Mutanen M., Hebert P. & Vila R. (2021) include P. tisiphone from Dibër county in the phylogentic tree.
Dapporto, Menchetti, Vodă, Corbella, Cuvelier, Djemadi., Gascoigne-Pees, Hinojosa, Lam, Serracanta, Talavera., Dincă & Vila R. (2022) documented COI gene diversity in Balkan Pseudochazara as part of The Atlas of Mitochondrial Diversity of Western Palearctic Butterflies.
(2023) provided further commentary on the IGV Atlas regarding P. tisiphone and designated the population in Dibër County as a distinct ESU.
(2025) assigned the population in Dibër County subspecies status as Pseudochazara tisiphone dibra ssp. nov. This new subspecies was registered in ZooBank.
Shortly after the subspecies status was assigned in (2025), Parmentier & Qirinxhi (2025) elevated the population in Dibër County to species level, naming it Pseudochazara misjai sp. nov. This new species was registered in ZooBank.
Supplementary material S2 provides the full references and hyperlinks, enabling further reading and verification of the publication details.
Conclusion
Eckweiler W. 2012. New discoveries of Pseudochazara mamurra amymone Brown, 1976 (Lepidoptera: Nymphalidae, Satyrinae). — Nachrichten des Entomologischen Vereins Apollo (NEVA) 33: 1–4. (url)
Gibbs M., Wiklund C. & Van Dyck H. 2010. Phenotypic plasticity in butterfly morphology in response to weather conditions during development. Journal of Zoology 283: 162-168. doi:10.1111/j.1469-7998.2010.00756.x
Gil-T F. 2017. Compared morphology and distribution of the taxa described of Pseudochazara williamsi (Romei, 1927) [= „Pseudochazara hippolyte“ Esper from Spain] Are they valid subspecies or only the result of phenotypic plasticity (ecological forms)? Atalanta Marktleuthen 48(1-4): 188-196. (url)
Gross F. 1973. Satyrus sintenisi auch in Europa, nebst Beschreibung einer neuen Unterart (Lep., Satyridae). — Entomologische Zeitschrift 83 (18): 211-214. (url)
Gross F. 1978. Beitrag zur Systematik von Pseudochazara-Arten (Lep., Satyridae). — Atalanta Würzburg 9: 41–103 (url)
Hesselbarth G., van Oorschot H. & Wagener S. 1995. Die Tagfalter der Türkei unter Berücksichtigung der angrenzenden Länder. — Selbstverlag Sigbert Wagener, Bocholt, Germany, 2201 p.
Koutsaftikis A. 1974. Recent Butterfly Records from Greece. Entomologist’s Record and Journal of Variation 86: 15-17. (url)
Kumar S., Stecher G., Li M., Knyaz C. & Tamura K. 2018. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Molecular Biology and Evolution 35: 1547-1549. https://doi.org/10.1093/molbev/msy096
Kusaba K. & Otaki. J 2009. Positional dependence of scale size and shape in butterfly wings: Wing-wide phenotypic coordination of color-pattern elements and background. — Journal of Insect Physiology 22(2): 175-183. doi.org/10.1016/j.jinsphys.2008.11.006
Lukhtanov V. 2007. Nymphalidae: Satyrinae. In: Global Butterfly Names Project. Global Butterfly Names – http://www.ucl.ac.uk/taxome/gbn/ (accessed on 07.iv.20)
Misja K. 1993. L'analyse faunistique des Lépidoptères diurnes de l'Albanie. — Biologia Gallo-hellenica 20(1): 157–168.
Misja K. 2005. Fluturat e Shqipërisë. Macrolepidoptera (Rhopalocera, Bombyces & Sphinges, Noctuidae, Geometridae). — Akademia e Shkencave e Shqipërisë, Instituti i Kërkimeve Biologjike,Tiranë, 247 p. [in Albanian] (url)
Misja K. & Kurrizi A. 1984. Resultats des recherche des papillons diurnes (Rhopalocera, Grypocera) de notre pays. — Buletini i Shkencave të Natyrës 12: 105–111.
Meteoblue (41.440°N, 20.200°E). Historical climate data for 41.440°N, 20.200°E. Meteoblue. (Accessed on 11.iv.2025), from https://www.meteoblue.com/nl/weer/historyclimate/climatemodelled/41.440N20.200E
Meteoblue (40.540°N, 20.770°E). Historical climate data for 40.540°N, 20.770°E. Meteoblue. (Accessed on 11.iv.2025), from https://www.meteoblue.com/nl/weer/historyclimate/climatemodelled/40.540N20.770E
Pamperis L. 2025. New Maps ETRS89 distribution (2025) with AOO 2x2 km and AOO 1x1 km. Before and after 2000. https://pamperis.gr/en/new-maps-of-distribution-etrs89-with-area-of-occupancy-1x1km/
Parmentier L. & Qirinxhi X. 2025. Unexpected cryptic diversity revealed through integrative analysis within isolated populations of the Graylings (Lepidoptera: Nymphalidae: Satyrinae) in the Western Balkans — Phegea 53(1) 22-38. (accessed via Figshare on 01.iii.2025, version 1)
Parmentier L. & Qirinxhi X. 2025. Unexpected cryptic diversity revealed through integrative analysis within isolated populations of the Graylings (Lepidoptera: Nymphalidae: Satyrinae) in the Western Balkans — Phegea 53(1) 22-38. (accessed via Figshare on 02.iii.2025, version 2)
Parmentier L. & Qirinxhi X. 2025. Unexpected cryptic diversity revealed through integrative analysis within isolated populations of the Graylings (Lepidoptera: Nymphalidae: Satyrinae) in the Western Balkans — Phegea 53(1) Supplementary material S1. Available at: (url) (accessed on 01.iii.2025)
Parmentier L. & Qirinxhi X. 2025. Unexpected cryptic diversity revealed through integrative analysis within isolated populations of the Graylings (Lepidoptera: Nymphalidae: Satyrinae) in the Western Balkans — Phegea 53(1) Supplementary material S3. Available at: (url) (accessed on 01.iii.2025)
Sašić M., Popović M., Cuvelier S., Đurić M., Franeta F., Gascoigne-Pees M., Koren T., Maes D., Micevski B., Micevski N., Mølgaard M., van Swaay C., Wynhoff I. & Verovnik R. 2015. Contribution to the knowledge of the butterfly fauna of Albania. — Nota lepidopterologica 38(1): 29–45. doi.org/10.3897/nl.38.8814
Takáts K. & Mølgaard M. 2016. Partial mtCOI-sequences of Balkanic species of Pseudochazara (Lepidoptera: Nymphalidae, Satyrinae) reveal three well-differentiated lineages. — Entomologica romanica 19: 21–40. (url)
Smith A. 1993. Tectonic significance of the Hellenic-Dinaric ophiolites. – Geological Society, London, Special Publications 76(1): 213-243. doi.org/10.1144/GSL.SP.1993.076.01.1
Taymans M., & Cuvelier S. 2025. A dynamic checklist of the Western Palearctic butterflies hyperlinked to the original descriptions at species, genus and family level (Lepidoptera, Papilionoidea). In Archives of Western Palearctic Lepidoptera 2025(1): 1-70. doi.org/10.5281/zenodo.14733224
Tennent J. 1996. The Butterflies of Morocco, Algeria and Tunisia. Gem Publishing Company, Oxfordshire, 217 p.
Tolman T. & Lewington R. 1997. Butterflies of Britain & Europe. — Harper Collins Publishers, London, 528 p.
Verovnik R. & Wiemers M. 2016. Species delimitation in the Grayling genus Pseudochazara (Lepidoptera, Nymphalidae, Satyrinae) supported by DNA barcodes. — Zookeys 600: 131–154. doi.org/10.3897/zookeys.600.7798
Wakeham-Dawson A. 1997. Discriminant analysis of androconia in the genus Pseudochazara de Lesse, 1951 (Lepidoptera: Satyridae). — Entomologist’s Gazette 48: 37-46. (url)
Wakeham-Dawson A. & Dennis R. 2004. A quantitative description of the androconia of British and Irish Hipparchia semele (Linnaeus, 1758) (Lepidoptera, Nymphalidae, Satyrinae). — Entomologist’s Gazette 55: 249-255. (url)
Wakeham-Dawson A. 2006. Description of wing androconia from some Pseudochazara de Lesse, 1951 (Lepidoptera: Nymphalidae, Satyrinae) type specimens in the Natural History Museum, London. — Entomologist’s Gazette 57: 99-107. (url)
Wakeham-Dawson A. & Kudrna O. 2000. A quantitative description of androconia from Staudinger’s Pseudochazara de Lesse, 1951 (Lepidoptera: Nymphalidae, Satyrinae) type specimens in the Zoological Museum of the Humboldt University of Berlin. — Entomologist’s Gazette 51: 75-81. (url)
Wakeham-Dawson A. & Kudrna O. 2006. Description of wing androconia from the lectotype of Pseudochazara caucasica (Lederer, 1864) (Lepidoptera: Nymphalidae, Satyrinae), with notes on the topotype wing androconia of related taxa. — Entomologist’s Gazette 57: 137-141. (url)
Wakeham-Dawson A., Kudrna O. & Dennis R. 2007. Description of androconia in the Palaearctic Asian Pseudochazara baldiva (Moore, 1865) butterfly species-group (Nymphalidae: Satyrinae) with designation of two lectotypes and reference to type and other material in the Natural History Museum, London. Nota Lepidopterologica 30(2): 211-223. (url)
Weiss J-C. 1980. Le genre Pseudochazara de Lesse en Europe et en Afrique du Nord. Description d’une sous-espèce nouvelle de Ps. hippolyte Esper (Lep.: Satyridae). — Linneana Belgica 8: 98–108. |