Lepidium didymum L., a new host plant for Pieris mannii (Mayer, 1851).
Is this new host plant facilitating the species' rapid northwestern expansion?
Submitted: 16.viii.2025 | Accepted: 07.ix.2025 | Published online: 30.ix.2025.
DOI: 10.5281/zenodo.17074253

Fig. 1. Pieris mannii ♂, Gullegem (Belgium), 12.ix.2021 (© Jacques Vervaeke)
Fig. 2. Pieris mannii ♀, Gullegem (Belgium), 28.ix.2022 (© Jacques Vervaeke)
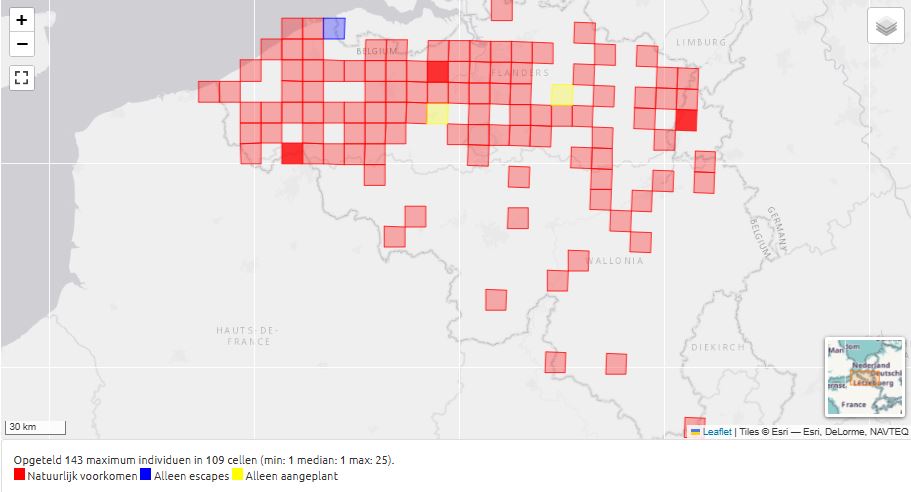 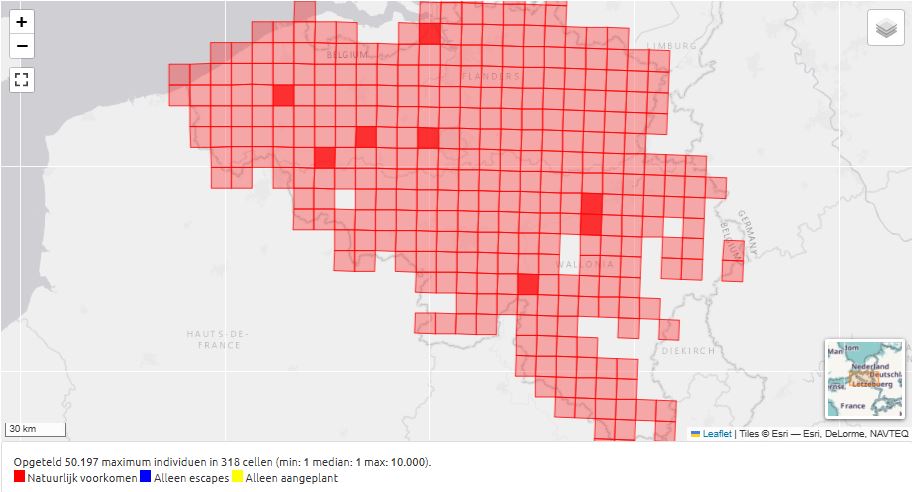 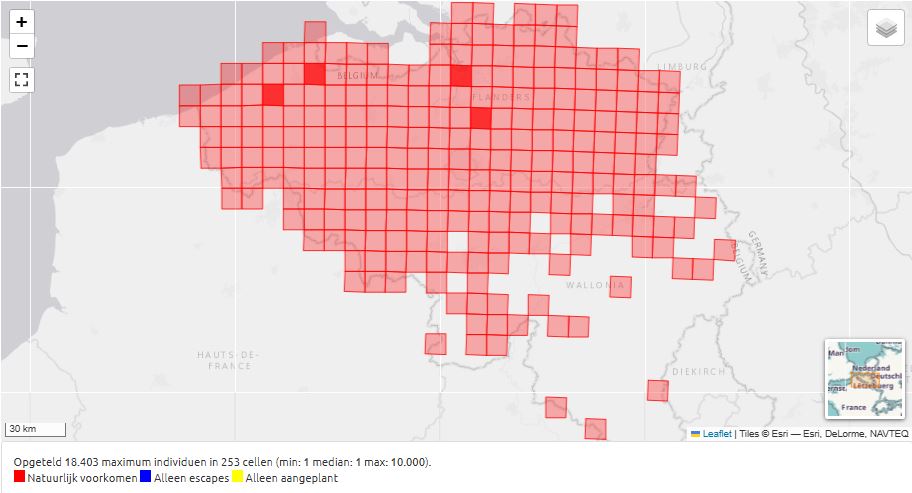
Fig. 17. Distribution of Brassica oleracea
Fig. 18. Distribution of Sinapis arvensis
Fig. 19. Distribution of Lepidium didymum
|