Archives of Western Palearctic Lepidoptera Open Source Research on Western Palearctic Lepidoptera |
AWPL 2025 (1): 71-77 |
|
|||
© 2025 Archives of Western Palearctic Lepidoptera.
All Rights Reserved. |

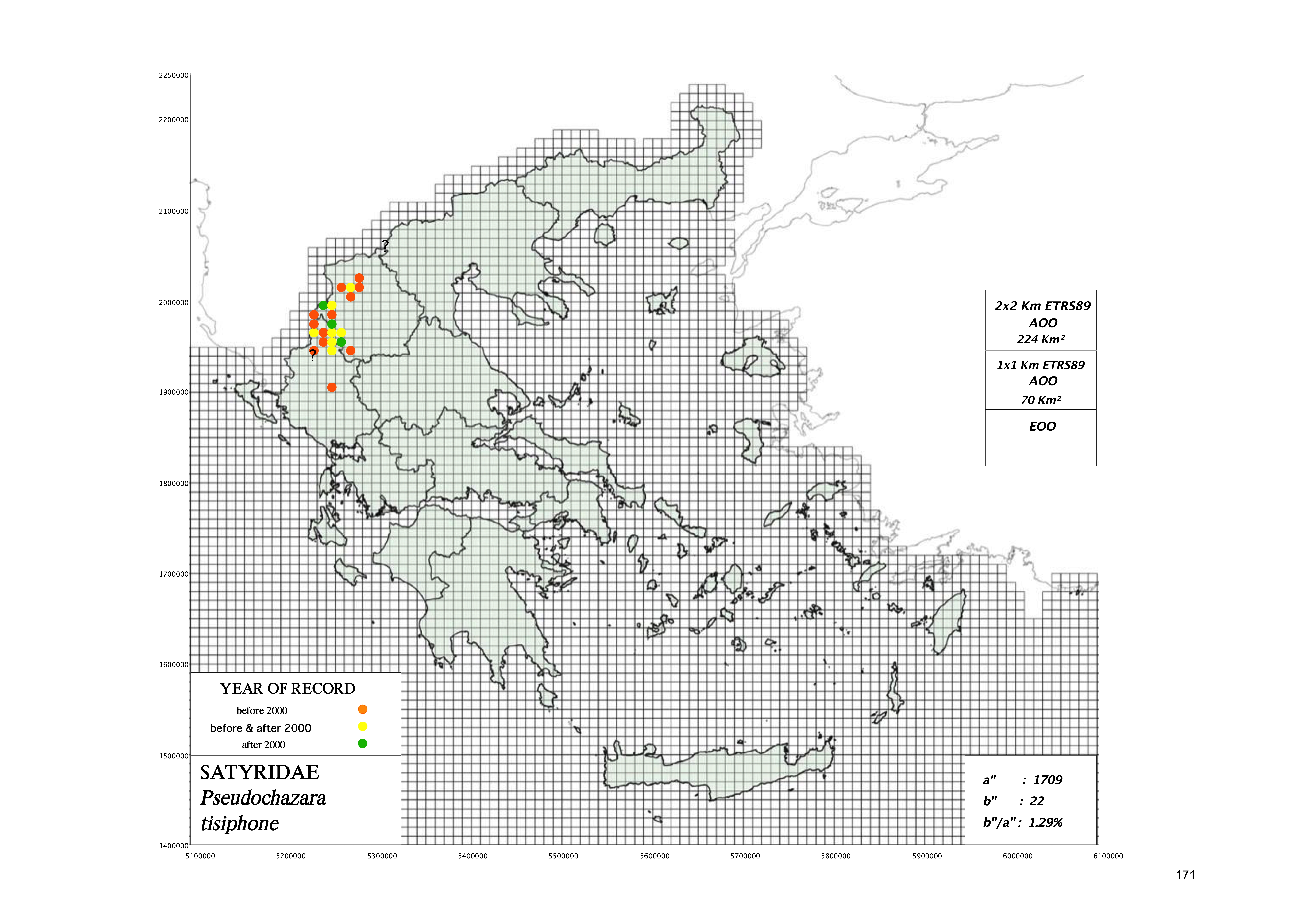
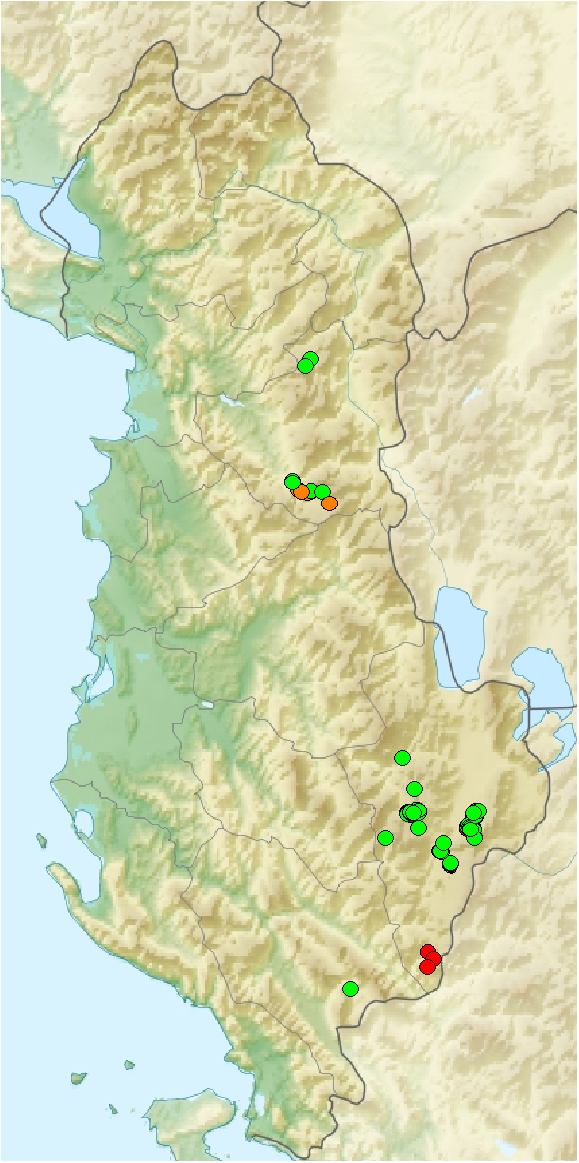
 Historical data ;
Historical data ;  Additional data from the 2018
Additional data from the 2018  New observations since the 2018
New observations since the 2018 


